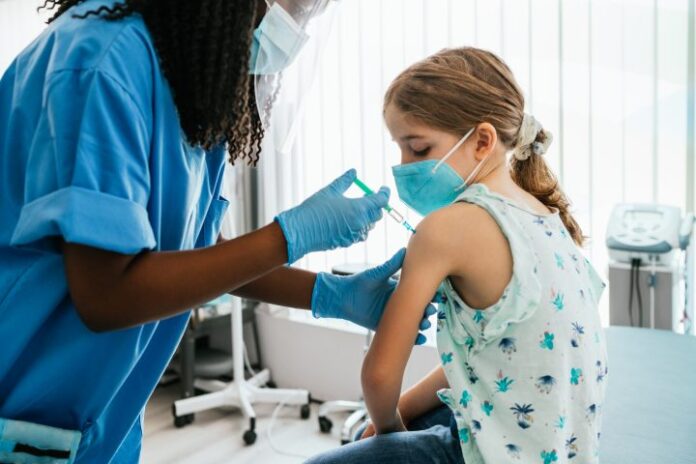
A second COVID-19 vaccine for Canadian children under the age of five years has been approved.
On Friday, Health Canada authorized Pfizer‘s pediatric COVID-19 shot for children aged six months to four years.
The agency had given the green light to Moderna’s vaccine for this age group in July.
In approving the the Pfizer-BioNTech Comirnaty COVID-19 vaccine for children under five, Health Canada said the benefits of this vaccine outweigh the potential risks in age group.
Three doses of Pfizer’s pediatric vaccine – of three micrograms each – have been authorized for the under-five age group, Health Canada said in a statement.
Trending Stories
Queen Elizabeth II, longest-reigning monarch in British history, dead at 96
Saskatchewan stabbing suspect Myles Sanderson dead after 4-day manhunt: RCMP
The first and second doses should be spaced out by three weeks, with a booster dose given eight weeks later.
Canada approves Pfizer’s COVID-19 booster for children 5 to 11 years old: Tam – Aug 19, 2022
The National Advisory Committee on Immunization (NACI) will issue recommendations on the use of Pfizer’s vaccine for the under-five group, Health Canada said.
The latest vaccine approval comes ahead of the fall season, when experts in Canada are anticipating another COVID-19 wave as children return to in-person classes and people gather indoors.
Last month, the first COVID-19 booster vaccine for children aged five to 11 years was approved in Canada.
In July, Health Canada approved Moderna’s Spikevax COVID-19 shot for the nearly two million children under the age of five.
© 2022 Global News, a division of Corus Entertainment Inc.