The Toronto family of four-year-old Michael Pirovolakis, the only child in Canada diagnosed with spastic paraplegia type 50, otherwise known as SPG50, has raised $3 million for gene therapy for their son.
“This could change his life. It can make him so much better,” said Terry Pirovolakis, Michael’s father.
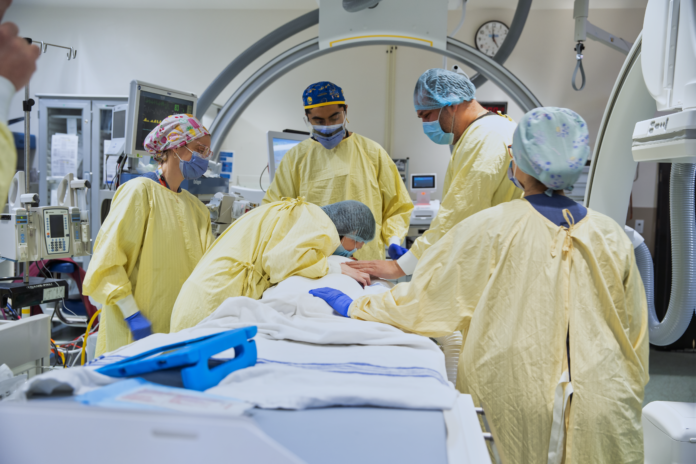
The Hospital for Sick Children (SickKids) had never before conducted a gene therapy clinical trial for only one patient. The clinical research team consulted with experts around the world and coordinated multiple teams and services to be able to provide such a novel treatment.
“Everybody pulled together and we just got to the finish line — maybe not as soon as we wanted, but we made it,” added Michael’s mother, Georgia Kumaritakis.
“June of last year we applied to Health Canada and to the FDA and we asked them if our package, in regards to our safety and efficacy for Michael’s gene therapy was OK. They came back with some modifications. We amended and December 30th, they gave us approval to dose Michael,” Terry said. “On March 24th, Michael was dosed with the gene therapy that we made.”
Researchers believe gene therapy has the potential to halt the progression of SPG50 and even reverse some of the damage.
“No one really knows. It’s unchartered territory,” Terry said. “We wait and we pray every day that Michael gets better.”
The family is now watching for signs that the treatment is working.
“The theory is that this would stop the disease, not that it would actually do anything further than that. So that’s what we’ve been told. I personally feel that if Michael was one month or six months of age, that this would have treated him and cured him. Unfortunately, Michael got the gene therapy at four years of age … All we’re trying to do is give him a better life,” Terry said.
Michael was one-year-old in 2019 when he underwent genome-wide sequencing at SickKids, which revealed he had two disease-causing variants in a gene called AP4M1. These variants result in SPG50, which is a progressive neurodegenerative disorder causing developmental delays, speech impairment, seizures, a progressive paralysis of limbs, and can be fatal.
It’s believed there are approximately 80 children around the world with SPG50, making the condition an ultra-rare disease.
Read more:
‘No cure’: Toronto family of toddler battling rare, new disease raises funds for research
“More and more now we’re running into situations where we’re finding conditions that there might be only a few other individuals in the world that have it,” said Dr. Jim Dowling, staff physician in the division of neurology and senior scientist in the genetics and genome biology program at SickKids, who led Michael’s clinical trial.
“That certainly was the case for Michael. So we were operating with a lot of unknowns, which creates a whole new set of stress and worry for the family.“
Michael’s treatment options were extremely limited and following his diagnosis, Michael’s parents immediately began raising money through a GoFundMe campaign to “Cure Michael, Cure SPG50.”
There have been countless fundraisers and the couple credits their East Toronto community with helping to get them to this point.
“First of all, we took out our life savings to start the programs because we couldn’t wait. But the second we came out with our story, we had people coming to our house, giving us envelopes of money, people organizing events. … We had beer stands, beer markets, galas, soccer tournaments, little kids doing lemonade stands on the side of the street,” Terry said.
“Generosity stepped up and that’s why 23,000 donations later, we have a therapy for Michael,” he added.
The family launched a charity, Cure SPG50, with the goal of “giving children affected by SPG50 a better tomorrow.”
The objective was to develop a gene therapy that could help Michael, and all others like him with SPG50. They worked with doctors and companies in Canada, the U.S. and elsewhere to coordinate the research, development and manufacturing of a gene replacement therapy that is meant to provide a normal version of the AP4M1 gene.
“The gene replacement therapy was administered into Michael’s spinal fluid through a lumbar puncture, or spinal tap. We used imaging technologies to guide the placement of the needle. The therapy was infused over a 10-minute period and then the needle was removed. Afterwards, Michael was kept on his stomach with his hips elevated for an hour in order to increase flow of the medicine to his brain,” Dowling said.
Successfully conducting the trial for Michael was not only a key milestone for him but also for achieving SickKids’ vision for precision child health, a movement to deliver individualized care for every patient, explained Dr. David Malkin, co-lead of the precision child health and director of the cancer genetics program at SickKids.
“What this demonstrated very nicely is we could have a journey for Michael where a genetic test was done which identified the gene alteration that caused the disease and then from that, we understand from the science that a drug can be developed, in this case a gene replacement treatment, and then that can be leveraged as a single patient for Michael,” Malkin said.
“That’s what the precision medicine is about. … We have a treatment that is specific for Michael’s particular disease.”
Doctors at SickKids are hopeful that learnings from Michael’s trial will help carve out a path for SickKids to explore innovative, precision-based treatment options for other patients with rare, genetic diseases.
“I think when this whole process started, if you would ask me, ‘Was it possible to develop a therapy for one or a small number of children that are affected with an ultra-rare condition?’ I don’t know that I would have said yes. But the fact that we’ve done this tells me we could do this for all the Michaels that we have at SickKids,” Dowling said.
Read more:
‘A deck of cards no one should be dealt’: Ontario siblings fight rare disease, hope for cure
Since receiving treatment, Michael now undergoes therapy for more than two hours every day at Canada’s largest children’s rehabilitation hospital, Holland-Bloorview in Toronto.
“We are cautiously optimistic. He lost a lot of the skills he had during the COVID break. His disease progressed dramatically without all those therapies in place. And so now we see hope. There is hope at the end of the tunnel,” his mother Georgia said.
“Along this journey, we’ve always said to ourselves, we love Michael no matter what. … If we get no therapy for Michael, it won’t change the way we love him. But if we can change his life for the better, amazing,” Terry said.
The family’s journey is not over.
“Even though in Toronto it’s been a journey to cure Michael, the story is Cure SPG50 and we made a promise to ourselves, me and my wife Georgia and our family that we would help as many kids as we could, not just Michael,” Terry said.
© 2022 Global News, a division of Corus Entertainment Inc.